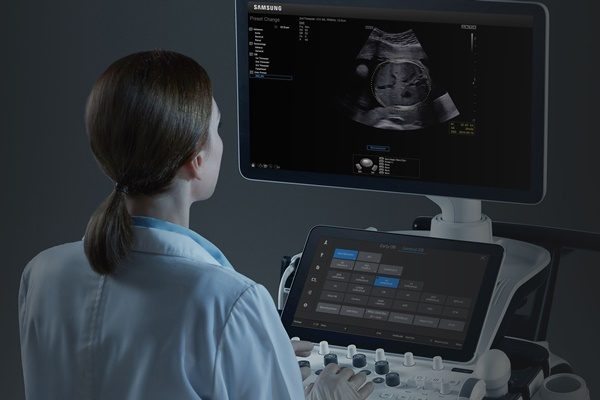
Samsung Medisonand Intel are collaborating on new smart workflow solutions to improveobstetric measurements that contribute to maternal and fetal safety and canhelp save lives. Using an Intel® Core™ i3 processor, the Intel® Distribution ofOpenVINO™ toolkit and OpenCV library, Samsung Medison’s BiometryAssist™automates and simplifies fetal measurements, while LaborAssist™ automaticallyestimates the fetal angle of progression (AoP) during labor for a completeunderstanding of a patient’s birthing progress, without the need for invasivedigital vaginal exams.
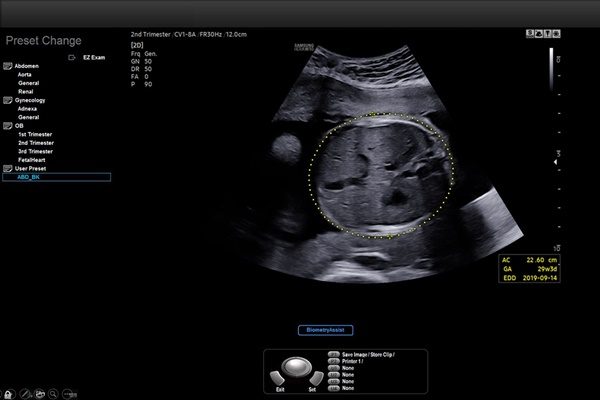
“Samsung Medison’s BiometryAssist is a semi-automated fetal biometry measurement system that automatically locates the region of interest and places a caliper for fetal biometry, demonstrating a success rate of 97% to 99% for each parameter1. Such high efficacy enables its use in the current clinical practice with high precision.”
–Professor Ja-YoungKwon, MD PhD, Division of Maternal Fetal Medicine, Department of Obstetrics andGynecology, Yonsei University College of Medicine, Yonsei University HealthSystem in Seoul, Korea
Why It’s Needed: According to the World Health Organization, about 295,000 womendied during and following pregnancy and childbirth in 2017, even as maternalmortality rates decreased. While every pregnancy and birth is unique, mostmaternal deaths are preventable. Research from the Perinatal Institute foundthat tracking fetal growth is essential for good prenatal care and can helpprevent stillbirths when physicians are able to recognize growth restrictions.
“At Intel, weare focused on creating and enabling world-changing technology that enrichesthe lives of every person on Earth,” said Claire Celeste Carnes, strategicmarketing director for Health and Life Sciences at Intel. “We are working withcompanies like Samsung Medison to adopt the latest technologies in ways thatenhance the patient safety and improve clinical workflows, in this case for theimportant and time-sensitive care provided during pregnancy and delivery.”
How It Works: BiometryAssist automates and standardizes fetal measurements inapproximately 85 milliseconds with a single click, providing over 97% accuracy1.This allows doctors to spend more time talking with their patients while alsostandardizing fetal measurements, which have historically proved challenging toaccurately provide. With BiometryAssist, physicians can quickly verifyconsistent measurements for high volumes of patients.
“Samsung isworking to improve the efficiency of new diagnostic features, as well ashealthcare services, and the Intel Distribution of OpenVINO library and OpenCVlibrary have been great allies in reaching these goals,” said Won-Chul Bang,corporate vice president and head of Product Strategy, Samsung Medison.
During labor,LaborAssist helps physicians estimate fetal AOP and head direction. Thisenables both the physician and patient to understand the fetal descent andlabor process and determine the best method for delivery. There is always riskwith delivery and a slowing progress could result in issues for the baby.Obtaining more accurate and real-time progression of labor can help physiciansdetermine the best mode of delivery and potentially help reduce the number ofunnecessary cesarean sections.
“LaborAssistprovides automatic measurement of the angle of progression as well asinformation pertaining to fetal head direction and estimated head station. Soit is useful for explaining to the patient and her family how the labor isprogressing, using ultrasound images which show the change of head stationduring labor. It is expected to be of great assistance in the assessment oflabor progression and decision-making for delivery,” said Professor Min JeongOh, MD, PhD, Department of Obstetrics and Gynecology, Korea University GuroHospital in Seoul, Korea.
BiometryAssistand LaborAssist are already in use in 80 countries, including the UnitedStates, Korea, Italy, France, Brazil and Russia. The solutions received Class 2clearance by the FDA in 2020.
Samsung Medison, located in Pangyo, was founded in 1985. It is a specialized company for ultrasonic diagnostic equipment and is a subsidiary of Samsung Electronics, a global company. Samsung Medison commercialized a Live 3D ultrasound diagnostic device in 2001 and was acquired by Samsung Electronics in 2011. Since then, it has been applied to medical devices in various fields ranging from radiology, obstetrics and gynecology to medical devices, introducing ultrasound products for diagnosis.
