PaxGenBio will participate in the 36th Korea International Medical & Hospital Equipment Show (KIMES 2021) which will run Mar. 18-21 to showcase MPCR-ULFA platform technology, its multi-rapid molecular diagnosis system.
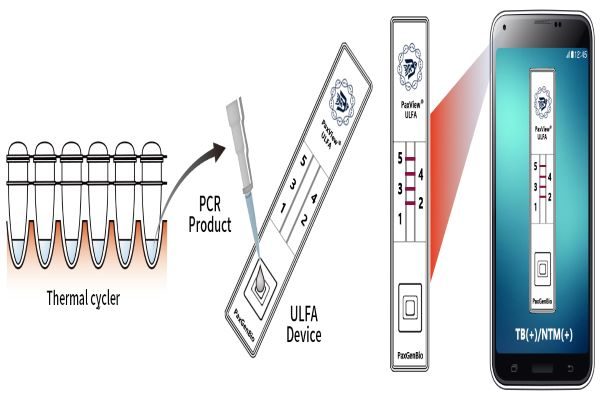
Multi-rapid molecular diagnosis system MPCR-ULFA (Multiplex PCR-Universal Lateral Flow Assay) platform technology consists of the multi-molecular diagnosis technology that amplifies multiple genes at the same time and the molecular rapid array technology that quickly and accurately analyzes the results of multiple amplified genes. Multi-rapid molecular diagnosis technology is a source technology capable of gene typing. Four patents including a US patent have been registered, and it is a quick and convenient molecular diagnosis kit that enables visual inspection of infection and genotyping.
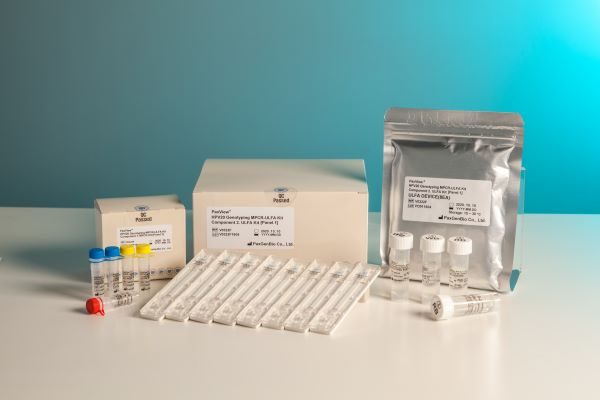
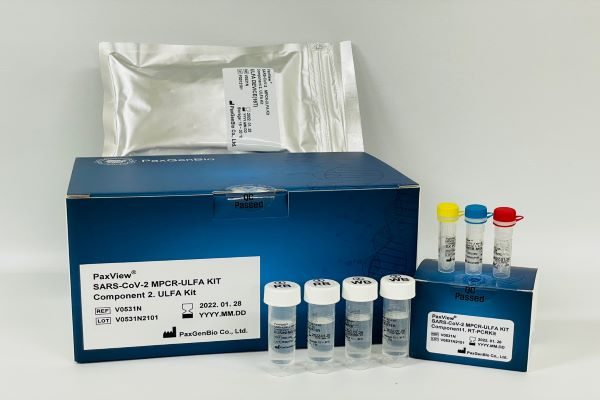
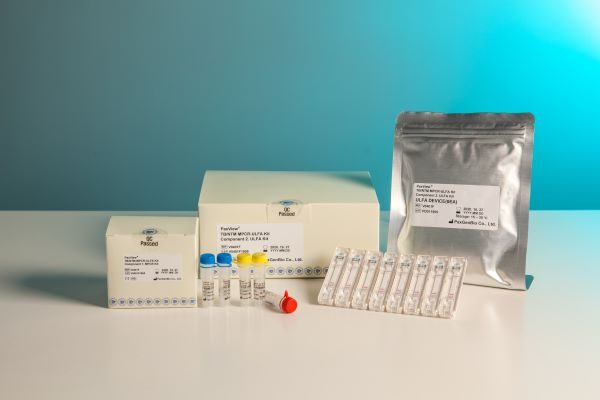
Based on the MPCR-ULFA platform technology, PaxGenBio developed PaxView® HPV 16/18/Others MPCR-ULFA Kit that can check the infection with human papilloma virus (HPV), especially the infection of type 16 and 18 with naked eye, PaxView® HPV 20 Genotyping MPCR-ULFA Kit that can analyze 20 types of HPV, PaxView® TB/NTM MPCR-ULFA Kit that can diagnose tuberculosis, PaxView® CT/NG/TP MPCR-ULFA Kit that can diagnose chlamydia, gonorrhea, and syphilis, and PaxView® SARS-CoV-2 MPCR-ULFA Kit that can diagnose COVID-19 infection.
PaxGenBio is participating in the project to combat tuberculosis in Indonesia, the country with the world's second highest prevalence rate of tuberculosis through the KOICA CTS project. Based on this, it will advance into overseas procurement markets such as WHO, and discover major Asian countries agencies and enter sales in the South American market, starting with tuberculosis diagnostic kits and HPV diagnostic kits for high-risk groups, and develop HPV type 20 genotypes and major STDs through business development with global partners and expand the sales network to diagnostic testing centers in Europe and China. The COVID-19 diagnostic kit is currently in discussion with the Philippines government for export.
KIMES is Korea's largest medical and hospital equipment show that has made steady growth with the development of the Korean medical industry, starting from its first in 1980. Currently, Korea’s medical industry has transformed into a technology-intensive high-tech industry such as medical information systems, ultrasound machines, imaging medical equipment, robotic medicine, AI, and rehabilitation medicine. KIMES is growing into a world-class medical exhibition while contributing to the advancement of the Korean medical industry. Exhibitors will showcase new technologies and new products in the medical industry, such as advanced hospital facilities, medical information systems grafted with IT technology, and automobile industry for rapid patient transportation.
→ Go to KIMES 2021 news page
