Ceres F&D, a company selected by the Incheon KOTRA Support Group's "I-BIO Customized DT Marketing Project," is a manufacturing company specializing in raw drugs that produce Vancomycin, Teicoplanin, and Tacrolimus, an immunosuppressant, mainly made after microbial fermentation and purification. It is equipped with cGMP facilities, is developing new biopharmaceuticals (Everlimus, Sirolimus), and is reborn as a global biopharmaceutical manufacturer with continuous R&D investments.

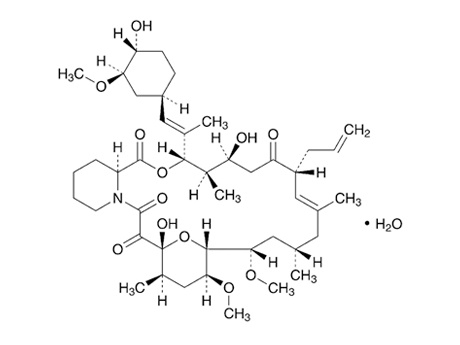
Tacrolimus, a major product of Ceres F&D, is a macrolide-based secondary product with immunosuppressive and antifungal activity and is used to suppress immune rejection in organ transplant patients, as well as to treat autoimmune diseases such as eczema.
Tacrolimus is a powerful and potential immunosuppressant and is known to have 10 to 100 times more immune suppression effects than cyclosporin, the most widely used immunosuppressant, and has reduced symptoms such as polyphagia, hemorrhoids, or high blood pressure in terms of side effects.
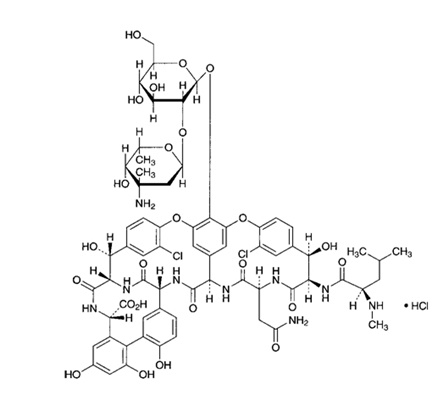
Vancomycin, an aseptic antibiotic injection, is widely used for bacterial diseases that are resistant to penicillin or ceparosporin-based antibiotics. In particular, vancomycin is mainly produced by industrial fermentation, and is administered intravenously to treat various infections (septic, skin infection, urinary tract infection, wound infection, etc.), and is also administered as an oral or ophthalmic agent to treat intestinal and eye diseases.
Sung-jin Joo, head of the Ceres F&D plant, said, "We received KGMP certification from the Ministry of Food and Drug Safety in 2007 under PIC/S (Fix) and WHO GMP, and we maintain the highest level of production and quality system. We have a lot of experience in due diligence from various overseas manufacturers such as India, the U.S., and CIS. "The production facilities include microbial fermentation facilities such as 50L, 500L, 4ton, 5ton, 10ton, 35ton, and refining facilities such as concentrators, reaction tanks and vacuum dryers."
Furthermore, he explained, "We are equipped with various analysis equipment such as HPLC, GC, FT/IR, Polarimeter, spectrophotometer, and TOC necessary to comply with the increasingly strengthened drug standards and manage them according to set procedures."
Regarding future plans, Joo said, "We are working on R&D to develop another immunosuppressant, Sirolimus, Tacrolimus capsule, and Everolimus, an anticancer drug," adding, "We are currently exporting products to India, Russia, CIS, and Southeast Asia, and we will focus on supplementing facilities and improving quality."
→ Click for more news on Incheon KOTRA Support Group ‘I-BIO Companies’
